Dec
1
Ultra High Pressures Might Become a New Storage Method for Hydrogen
December 1, 2009 | 3 Comments
Filled with enthusiasm, scientists at the Carnegie Institution have found for the first time that high pressure can be used to make a unique hydrogen-storage material. Led by Maddury Somayazulu, the Carnegie team found that the normally unreactive, noble gas xenon combines with molecular hydrogen (H2) under pressure to form a previously unknown solid material with unusual bonding chemistry. The experiments are the first time these elements have been combined to form a stable compound. The discovery debuts a new family of materials, which could boost new hydrogen storage technologies.
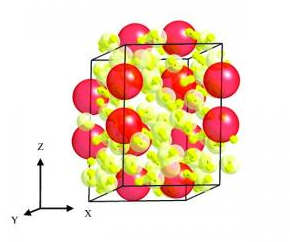
Somayazulu explains, “Elements change their configuration when placed under pressure, sort of like passengers readjusting themselves as the elevator becomes full. We subjected a series of gas mixtures of xenon in combination with hydrogen to high pressures in a diamond anvil cell. At about 41,000 times the pressure at sea level (1 atmosphere), the atoms became arranged in a lattice structure dominated by hydrogen, but interspersed with layers of loosely bonded xenon pairs. When we increased pressure, like tuning a radio, the distances between the xenon pairs changed – the distances contracted to those observed in dense metallic xenon.” Tests pressures went up to – 255,000 atmospheres.
This isn’t sounding practical, yet. Over a quarter million atmosphere is squeezing things pretty tight.
Somayazulu and his colleagues’ paper is published in the November 22, 2009, advanced online publication of Nature Chemistry.
Xenon has some intriguing properties, including its use as an anesthesia, its ability to preserve biological tissues, and its use in lighting. Xenon is a noble gas, which means that it does not typically react with other elements.
The Carnegie team imaged the compound at varying pressures using X-ray diffraction, infrared and Raman spectroscopy. When they looked at the xenon part of the structure, they realized that the interaction of xenon with the surrounding hydrogen was responsible for the unusual stability and the continuous change in xenon-xenon distances as pressure was adjusted from 41,000 to 255,000 atmospheres.
Houdini hydrogen has been intensely studied in hopes of finding a way to contain it. So with the news release a question comes up, why is this compound so stabile? Przemek Dera, the lead crystallographer who looked at the changes in electron density at different pressures using single-crystal diffraction said, “We were taken off guard by both the structure and stability of this material.” As electron density from the xenon atoms spreads towards the surrounding hydrogen molecules, it seems to stabilize the compound and the xenon pairs.
Now this writer is thinking and perhaps you as well, that the equipment needed to accomplish forming the material and then the matter of recovering the hydrogen hasn’t been addressed. Plus Somayazulu himself offers, “But by understanding how it works in this situation, researchers can come up with lighter substitutes.”
Russell Hemley, director of the Geophysical Laboratory and a co-author says, “It’s very exciting to come up with new hydrogen-rich compounds, not just for our interest in simple molecular systems, but because such discoveries can be the foundation for important new technologies. This hydrogen-rich solid represents a new pathway to forming novel hydrogen storage compounds and the new pressure-induced chemistry opens the possibility of synthesizing new energetic materials.”
This is quite intriguing. Hydrogen, the smallest anything atom in a universe made up of atoms is just a devil to contain – unless locked up in a molecular bond such as water. Humanity seems determined to chase the goal of an economical way to store hydrogen without it simply escaping away.
The Carnegie team’s effort is noteworthy in that hydrogen is bound, by pressure if not molecularly to another atom. The idea may prove to have merit if lighter elements can be used, but realistically those pressures have to come down, way way down for any commercial use an be considered. Its just simpler to break hydrogen out of water or natural gas and use it right away than try to store it.
But a solid form of hydrogen is of great interest, and may well point the way to better forms of hydrogen storage. It might even set off a flurry of other pressure experiments that might turn up other discoveries of unexpected principles of value.
Comments
3 Comments so far


There’s a BLEVY to end them all!
Overcoming the storage of hydrogen in a compact way is the catalyst for growing a hydrogen economy. All other issues relating to the energy conversion to produce hydrogen would be viewed differently with viable high density energy storage. The Pivotal engine is the low cost high power density and high efficiency solution to complete the package for viable hydrogen power. http://www.pivotalengine.com
Keep posting stuff like this, I really like it