Aug
5
The Hydrogen Economy Might Have Gotten Real Legs
August 5, 2008 | 11 Comments
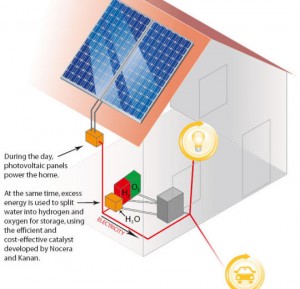
I have been quite unimpressed by the “hydrogen economy” concept because of the difficulty in storing and transporting the smallest atom. I can live with the other attributes as careful engineering can cope. I still have reservations about regular folks handling such a volatile fuel. This is moderated by the reports out of MIT from Professor Daniel Nocera and Matthew Kanan. These men have allayed my worst fears by thinking through a closed loop system that would not have normal people handling hydrogen. The loop is a new way to crack the hydrogen out of water and within a day feed it into a fuel cell for generating electrical power. I can live with that, barely.
Now not to enthuse or frighten folks, but Professor Nocera is quoted as saying, “I’m open-sourcing this to let everybody run with it. My plan is that when people see it, they’ll see it’s easy to do and they’ll start working it.”
The story begins in the effort by many in chemical research to create an artificial photosynthesis, so getting the plant kingdom’s technology into humanity’s hands. The MIT team’s results have gathered acclaims like “This discovery is simply groundbreaking,” from Karsten Meyer, a professor of chemistry at Friedrich Alexander University, in Germany.
The innovation it seems from looking at this after the fact is the research was driven not to electrolysis of water, but to drive off the oxygen as in plants leaving the hydrogen behind. From the news information that’s out the team developed a catalyst that’s powered by electricity, which does drive off the oxygen and leave hydrogen behind in ion form. This solves the problem of splitting out hydrogen and getting an increasingly rich stock of hydroxide left behind. It also solves the separation issue of the two gasses.
Then as Professor Nocera put it in the (pdf) podcast, (media) podcast. “You take water plus these catalysts and light from the photovoltaic and you make hydrogen and oxygen. You store the hydrogen and oxygen, then when you combine it back over, say, a fuel cell, you get water back and electricity out. So in this sort of design, you don’t need to worry about where you’re going to get the water from except the initial time. You get the initial water, then you’re in a closed loop, and it’s just humming away with light as an input from a photovoltaic. You’re taking water to hydrogen and oxygen and then hydrogen and oxygen back to water.” You might note that the electrical input could be from any source.
Using little more than a conducting material Nocera is making up a solution of cobalt ions and phosphate ions and forming a film on the conductor making his electrode. The conductor Nocera uses is indium 10 oxide glass. Nocera then explains, “Once you make that electrode, you can take it out. And you don’t need the cobalt anymore but it’s good to have the phosphate around. And the reason for that is, remember when we started it was just a clean piece of glass, and then when we put the positive potential on it, it oxidizes cobalt. It takes it from what’s called cobalt 2+ to cobalt 3+ and the cobalt 3+ and the phosphate form this thin film. Now in the natural cycle of this catalyst working, it goes back to cobalt 2+ and then it can redissolve. But then when the electrode’s under a positive potential it goes back to cobalt 3+ and then the phosphate in solution will capture it and bring it back to the electrode. And so we call that self-healing or a repair mechanism. So what you can do is make the catalyst out of cobalt and phosphate, get your thin film, it’s like a darkish, blue-black film on the conducting glass, take the conducting glass out and put it on a shelf. Then when you’re ready to make O2 you can take that conducting glass, put it into a solution of phosphate, just phosphate and water now, phosphate’s a mineral anion. And then the thing just hums away making O2. And the reason you want that phosphate is because in the natural cycle of the catalyst performance, the cobalt can redissolve in solution and then the phosphate escorts it back to the electrode.”
| Watch Daniel Nocera explain how his catalyst can be used to store sunlight. |
That leaves you with an ionic hydrogen rich water that can have the hydrogen extracted using a platinum catalyst. Professor Nocera expects that within months there will be a working alternative as he has one working experimentally now in preparation for the next paper that could tie the whole process into a neat whole.
The title has the word “might” as there is a missing element – efficiency. Just how much hydrogen is the new process going get vs. the electrical input? That goes unsaid. But by no means is this a non-event, the new take, to get out the oxygen then extract the hydrogen is a worthwhile path that has just seen the first concrete results. If it is efficient, yielding high returns of hydrogen the prospects are stunning. Imagine you are an organic chemist with an abundant and cheap supply of hydrogen.
You might revolutionize oil refining, or simply use coal at the mine to make methane (natural gas) or use hydrogen in oil reservoirs for enhanced recovery, or find a way to extract heavy oil, tar sand oil, oil shale and other sources into a clean burning, pipeline transportable fuel that can be in business, homes and vehicles.
Might. Yet the prospects in this field are amazing with incredible potential. But there is an early issue yet to resolve – Nocera is quoted as above in going to open source the technology, while the MIT lawyers have filed patents. There had to be a problem! But keep in mind; the MIT team is only the first to publish. For the dreamy but possible version of the future try the Science Daily version of the story. The future looks good.
Comments
11 Comments so far



Nice Site layout for your blog. I am looking forward to reading more from you.
Tom Humes
[…] See original here: The Hydrogen Economy Might Have Gotten Real Legs […]
Thanks for explaining that beyond the press release’s illiterate hype! I hate coal as much as the next air breather, but there seems something workably utilitarian, if discriminatory, in burning the coal right at the mine and exporting a product the rest of us can live with. Hey, if they want their jobs so badly…
Would it be possible, starting with this new technology, to deliver a home based solution for producing and storing methanol? That sounds like a preferable fuel to hydrogen for many reasons, so what would prevent us from taking the extra step? One way or another, our future phones, laptops, and lawnmowers must be topped off, might as well make the juice at home.
Jon,
Its under development One such site is: http://almostfreefuel.com/methanol.html I haven’t looked into it in detail yet. Fuel cells are on the way. The DOD has them in use now. We may make our own, my grandfather did during the Great Depression in both a Model T and Model A. Modern techniques are more efficient in using the feedstocks. Methanol should be cheap. Expect government to resist as making your own would deprive them of taxes.
2008: The Year of (Un)Sustainable Biofuel …
Last year, I used a special run of AltEnergyStocks.com’s new Cleantech News (CTN) aggregator to bring you the ten stories of 2007 which bloggers found most interesting or controversial. Continuing the tradition, below are the ten stories of 2008…
Great site. A lot of useful information here. I’m sending it to some friends!
Hey all.. I am quite new to running a blog world and that i already been doing a bit of searching to obtain ideas. Your own wordpress blog certainly offers assist me to. Thank you for which!
I REALLY liked your post and blog! It took me a minute bit to find your site…but I bookmarked it. Would you mind if I posted a link back to your post?
This post makes a lot of sense !
I’ve just started off a blog, the knowledge you give on this site has aided me extremely. Thank you for all your time & work.
Great read. Thanks for the info!