Jun
23
A Better Way to Make Carbon Pay
June 23, 2008 | 5 Comments
It’s been over 110 years since William Jacques, an American electrical engineer and chemist, first described the Direct Carbon Fuel Cell back in 1896 in his original patent application. The DCFC was explored over the years in efforts to overcome the problems until the 1970s when SRI International of Menlo Park California verified the original design by Jacques would generate electricity. Proof in hand the SRI group innovated several design modifications but the DCFC remained impractical. The DCFC has been picked up again by several groups since the mid 90s and the latest advances in materials science, other fuel cell developments and basic electrochemistry have sparked plans to have commercial viability in 5 years and large-scale installations in 20.
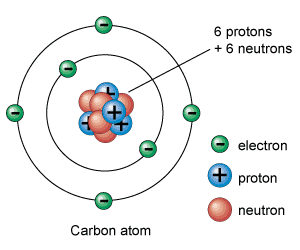
What makes the DCFC so interesting? All that power potential in a very dense fuel base. Hydrogen has one proton and one electron, the smallest density possible. Carbon has 6 protons and in stabile form 6 electrons making it 6 times as dense. Carbon can be part of molecules from gases to liquids to solids. Its very handy stuff. Its everywhere and easily manipulated.
That makes the DCFC an intensely interesting prospect. Moreover, running a fuel cell is fundamentally different from burning a fuel. The products from the reactions are not fly ash, CO2 and host of complex airborne chemicals. A fuel cell while in the carbon scenario would be quite hot, the oxidation would be taking place in a circumstance very different from combustion. Burning is a raw form of oxidation, where fuels and oxygen are ignited and react releasing heat. A fuel cell forms an electrochemical reaction that oxidizes through the use of an electrolyte – ignition and flames with the resultant heat is avoided. The yield is directly to electrical potential, skipping the heat, transfer to water to make steam and on to a turbine and then a generator. Each step avoided raises efficiency. Burning is just the simplest way to extract the chemical energy in a fuel.
Obviously making the jump to fuel cell would greatly reduce the amount of fuel needed to generate a given amount of power. The three most important factors in considering a fuel cell are the cell’s own thermodynamic efficiency. Fuel cells need to run at temperatures that optimize the fuel, electrolyte and physical unit properties. The choice of fuel is important as the more reactant in a volume, the better, as we noted in comparing the hydrogen electron count with carbon. The last is the voltage efficiency or answering the “pressure” of the cell’s output. In all three basic factors, carbon fuel cells look good.
That makes the news of late last week noteworthy as John Irvine and his colleagues at the University of St Andrews announced and published results of their run of a hybrid DCFC that has two types of electrolytes. With a binary system to process fuel, the oxidation took place both on the electrode surface but also in the carbon/electrolyte mix. That makes for some interesting power production results.
Carbon is sure to be with us for fuels and energy production for a long time. A carbon fuel for a fuel cell can optimize the value of a carbon’s high density from such a wide array of sources from waste materials, biomass, coal, coke left over from petroleum refining and most anything that has carbon in appreciable proportion. Processing feedstocks to prepare for fuel cell use itself offers opportunities.
To quote Mr. Irvine, “Carbon fuels offer high efficiency of conversion and if implemented in the correct way can yield two to three times the amount of energy for a given amount of fuel compared to thermal generation.” That’s like using half or a third as much fuel to do the same work.
A hat tip to Al Fin who first posted about the St Andrews breakthrough and started the hunt for the current art in DCFCs. Here are some links for digging in deeper:
Chemical Technology has a brief look at the St Andrews work with some comments and observations.
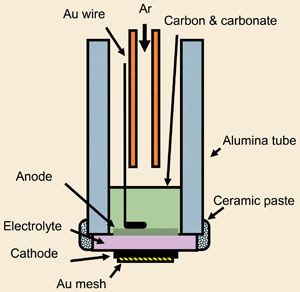
Energy & Environmental Science has a brief about the published paper that offers a quick look at the chemistry and basic chemistry apparatus graphic. Energy & Environmental Science also has the paper available to anyone (A Boon To Interested Persons!) as an html download that can be read on any Internet connected computer.
This is a lot to add to a Monday blog post, but when considered, a DCFC at commercial scale would go far to enhancing electrical power generation worldwide. It plays into conservation, reducing CO2 emissions, participating responsibly in the biosphere and planetary carbon cycle and offers basic roles to lots of participants in providing basic feedstocks. It would irrevocably reverse the domination of OPEC and the Axis of Oil in hydrocarbons as the primary source of fuels substituting thousands of alternative providers. That may also be a disaster for speculators, as a widely diffused source of feedstocks would be locally priced instead of being a single worldwide commodity.
Comments
5 Comments so far


Do you people have a facebook fan page? I looked for one on twitter but could not discover one, I would really like to become a fan!
Awesome post. I so good to see someone taking the time to share this information
Thanks for posting. Good to see that not everyone is using RSS feeds to build their blogs 😉
Interesting read, perhaps the best article iv’e browse today. We learn everyday cheers to you!
This is awesome information! I have to thank you for providing so much insight into this subject. I am so happy I found your article.