May
8
Is Methanol the Up and Coming Alcohol For Fuel?
May 8, 2008 | 5 Comments
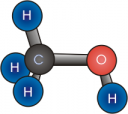
Methanol is the smallest of the alcohols, with one carbon atom, four hydrogen atoms and an oxygen atom, which is parked between the carbon and one of the hydrogen atoms. The oxygen atom is the only difference between methanol and methane the main part of natural gas. Methanol also known as wood alcohol, is a comparatively easy molecule to make from biomatter. Like methane, it burns cleanly, as the hydrogen to carbon ratio is at 4:1 and it has that oxygen atom that conveniently helps keep methanol liquid and increases its resistance to ignition. Good stuff, if not very high energy in density, as shown by its long history as a racing fuel.
The breakthrough might be using methanol in a fuel cell rather than in an internal combustion engine. Like other fuels methanol isn’t going to overcome the inherent disadvantage of the IC engine efficiency, but applied to a fuel cell the efficiency may get high enough that the power we are accustomed to and the ranges we expect may still be available.
A fuel cell using methanol would handle a chemical reaction called electro-oxidation in which a catalyst would be needed to accelerate the process up to output rates that make sense. The idea is to generate electrical output instead of ignition into flames. Compared to straight hydrogen, methanol offers being a liquid, much higher energy density, safer to handle, much lower pressures in storage and in an accident a fire rather than a concussive explosion. In a fuel cell system, the high heat mechanical apparatus wouldn’t likely be present for an ignition source.
The problems that have held up methanol as a fuel cell feedstock is the need for a great deal of rare platinum for the catalyst and the output of carbon monoxide rather than carbon dioxide. With all the complaining about CO2 it is still a better and life sustaining molecule over the poisonous and near valueless CO. These issues have kept methanol deep into research.
The world has its new hero. Jose E. Barranco is a newly minted PhD whose thesis “Development of New Metallic Materials of an Amorphous Nature for Use in Direct Methanol Fuel Cells” was awarded “Excellent Cum Laude” unanimously at Universidad del Pais Vasco/Euskal Herriko Unibertsitatea (aka University of the Basque Country).
Dr. Barranco’s thesis is based on his work in making alloys including nickel, niobium, antimony, ruthenium and others with platinum as low as 1% that have the capability to act as a methanol fuel cell and convert the carbon monoxide into carbon dioxide. The work led to efforts to increase efficiency. The amorphous structure is a result of efficiency effort. Dr. Barranco found that an amorphous structure for the platinum alloy increased its conductivity and it experienced less corrosion. The operational capacity increased 80 to 100 times higher than platinum in its crystalline structure.
Dr. Barranco went even further in choosing to change the form of the alloy to very fine power that he essentially “spray painted” onto the fuel cell’s membrane. This raised the operating capacity of the catalyst to “9-13 times” which forms a fuel cell better than 50% more efficient than a crystalline platinum fuel cell.
That’s the good news. Here is what may prove to be bad news. Dr. Barranco’s work is under the jurisdiction of the Alcohols Oxidation Fuel Cell Research department at the Industrial Chemistry and Electrochemical Engineering Laboratory of the Polytechnic University in Donostia-San Sabastian. The research there is lead by Dr. Angel Rodriguez Pierna whose aim seems to be to “achieve a methanol fuel cell solely and totally devised and developed by the laboratory.”
After all fact checking I find these highly plausible innovations under the purview of one administrative fellow. Methanol is a good prospect for an alternative biomass derived fuel that plays well with planetary biosphere carbon recycling and is a one carbon atom molecule, which brings four hydrogen atoms to the reaction. This is the sort of thing that makes the high energy, green revolution folks glow. In fuel cell use it would electrify drive trains and may drive down the costs of replacing the personal car fleet while displacing precious petroleum oil for burning to energize cars. It could be a very big deal indeed.
Methanol is easy to make, one has to be careful though, even when making ethanol one can lose control and get methanol by mistake.
But, its under the control of just one man.
That’s enough to make one really uneasy about Dr. Barranco’s future. I wish these men well, I just hope the innovation gets out for replication and a much wider field of research soon. It looks like a “home run,” I just hope the stadium has an audience that matters and stays around long enough to matter.
Comments
5 Comments so far



[…] Yesterday we looked at a method of releasing hydrogen from methanol. Today we’ll look at releasing hydrogen from formic acid. […]
[…] week before last saw the Basques offer they have a fuel cell that can be fueled by methanol. With in a day I had received multiple notices that MIT in Massachusetts and Sharp of Japan were […]
[…] hydrogen atoms. The oxygen atom is the only difference between methanol and methane the main parthttps://newenergyandfuel.com/http:/newenergyandfuel/com/2008/05/08/is-methanol-the-up-and-coming-alco…AFX UK Focus 2008-05-27 11:30 Indonesia signs oil & gas contracts worth 201 mln with Eni unit, […]
[…] of the IC engine efficiency, but applied to a efficiency cell the bloomington may get …https://newenergyandfuel.com/http:/newenergyandfuel/com/2008/05/08/is-methanol-the-up-and-coming-alco…Fuel Testers -Ethanol Fuel Test Kits -Protect your engines from …Fuel-Testers ethanol articles and […]
[…] MIT has a new material for the fuel cell membrane that may become a huge cost reducer, the Spanish are determined to top everyone’s efforts with a catalyst added to the cell to assist in th… that displaces using the expensive mineral platinum. The Japanese are racing and partnering with […]