Aug
2
Fuel From The Air’s CO2
August 2, 2016 | Leave a Comment
Argonne National Laboratory and the University of Illinois at Chicago researchers have found a way to convert carbon dioxide into a usable energy source using sunlight. In a spectacular PR coup the Argonne lab managed to get major media attention, and the University of Illinois press lease even made Google news, but we’ll have a more technical look.
Argonne chemist Larry Curtiss, the Argonne National Lab corresponding author of the study said, “On its own, it is quite difficult to convert carbon dioxide into something else,” noting the one of the chief challenges of sequestering carbon dioxide is that it is relatively chemically unreactive.
The chemical inertness of CO2 renders many electrochemical and photochemical conversion processes inefficient. The research team found that tungsten diselenide nanoflakes show a current density of 18.95 milliamperes per square centimeter, CO faradaic efficiency of 24%, and CO formation turnover frequency of 0.28 per second at a low overpotential of 54 millivolts.
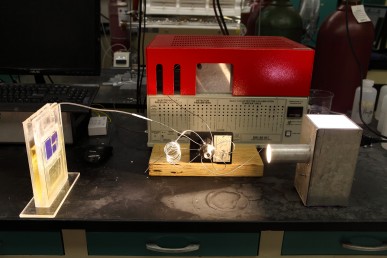
CO2 To Fuel Lab Unit at the University of Illinois at Chicago. Image Credit: University of Illinois at Chicago. Click image for the largest view.
To make carbon dioxide into something that could be a usable fuel, Curtiss and his colleagues needed to find a catalyst – a particular compound that could make carbon dioxide react more readily. When converting carbon dioxide from the atmosphere into a sugar, plants use an organic catalyst called an enzyme. The researchers on the other hand used the metal compound tungsten diselenide, which they fashioned into nanosized flakes to maximize the surface area and to expose its reactive edges.
While plants use their enzyme catalysts to make sugar, the Argonne researchers used the tungsten diselenide to convert carbon dioxide to carbon monoxide.
Amin Salehi-Khojin, assistant professor of mechanical and industrial engineering at UIC and senior author on the study said, “The active sites of the catalyst get poisoned and oxidized.” The breakthrough, he explained, was to use an ionic fluid called ethyl-methyl-imidazolium tetrafluoroborate, mixed 50-50 with water.
Although carbon monoxide is also a greenhouse gas, it is much more reactive than carbon dioxide and scientists already have ways of converting carbon monoxide into usable fuel, such as methanol.
Argonne physicist Peter Zapol, a co-author of the study explained, “Making fuel from carbon monoxide means traveling ‘downhill’ energetically, while trying to create it directly from carbon dioxide means needing to go ‘uphill.”
The setup for the reaction is sufficiently similar to nature that the research team was able to construct an “artificial leaf” that could complete the entire three-step reaction pathway.
In the first step, incoming photons – packets of light – are converted to pairs of negatively-charged electrons and corresponding positively charged “holes” that then separate from each other. In the second step, the holes react with water molecules, creating protons and oxygen molecules. Finally, the protons, electrons and carbon dioxide all react together to create carbon monoxide and water.
Then the team applied this catalyst in a light-harvesting artificial leaf platform that concurrently oxidized water in the absence of any external potential. When light of 100 watts per square meter – about the average intensity reaching the Earth’s surface – energizes the cell, hydrogen and carbon monoxide gas bubble up from the cathode, while free oxygen and hydrogen ions are produced at the anode.
Zapol explained the driver for their work this way, “We burn so many different kinds of hydrocarbons – like coal, oil or gasoline – that finding an economical way to make chemical fuels more reusable with the help of sunlight might have a big impact.”
Towards this goal, the study also showed that the reaction occurs with minimal lost energy – the reaction is very efficient. “The less efficient a reaction is, the higher the energy cost to recycle carbon dioxide, so having an efficient reaction is crucial,” Zapol said.
According to Curtiss, the tungsten diselenide catalyst is also quite durable, lasting for more than 100 hours – a high bar for catalysts to meet.
This is an extraordinary research effort. Just keep in mind this technology is still lab work and no mention is made beyond more than 100 hours of catalyst activity, about scaling up the technology or the economic prospects. The team’s artificial leaf reduced CO2 on one side while a cobalt catalyst oxidized water on the other side.
What’s missing in the public domain is the making fuel step. The syngas of carbon monoxide and hydrogen isn’t especially useful by and in itself, its more of a precursor. There are a few possibilities, which lead us back to the PR success.
Nothing in the press releases or study abstract supports some bizarre fragments in the major media. It must be said that way as the researchers and the press release work are exemplary. But its another powerful example of the media misleading from ignorance, incompetence, or perhaps some nefarious motive.
It is great news all by itself, no one had to say, “The result: an artificial leaf that turns CO2 into fuel, “at a cost comparable to a gallon of gasoline” could render fossil fuel obsolete, according to the researchers,’ in a quote attributed to – no one.
It would have been nice to see how much syngas per unit of time at a normal solar input would yield. Meanwhile, lets hope the team stays with it. The catalyst will needs to run in the many thousands of hours at decent level of productive efficiency.

