Jan
13
Steps To Make Fuel From the Atmosphere’s CO2
January 13, 2016 | 1 Comment
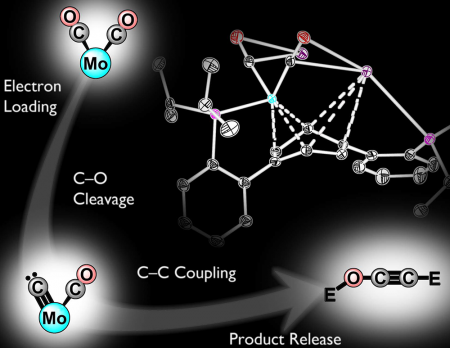
C1 to C2: Connecting carbons by reductive deoxygenation and coupling of CO. Image Credit: Kyle Horak and Joshua Buss, Caltech. Click image for the largest view.
The findings, published in the journal Nature provide a foundation for the development of technologies that may one day help recycle the atmospheric accumulation of CO2 by converting it back into fuel. Although methods exist to transform CO2 into CO, a crucial next step, the deoxygenation of CO molecules and their coupling to form C-C bonds, is more difficult.
In their study, Agapie and Buss synthesized a new transition metal complex – a metal atom, in this case molybdenum, bound by one or more supporting molecules known as ligands – that can facilitate the activation and cleavage of a CO molecule. Incremental reduction of the molecule leads to substantial weakening of the C-O bonds of CO. Once weakened, the bond is broken entirely by introducing silyl electrophiles, a class of silicon-containing reagents that can be used as surrogates for protons.
This cleavage results in the formation of a terminal carbide – a single carbon atom bound to a metal center – that subsequently makes a bond with the second CO molecule coordinated to the metal. Although a carbide is commonly proposed as an intermediate in CO reductive coupling, this is the first direct demonstration of its role in this type of chemistry, the researchers said. Upon C-C bond formation, the metal center releases the C2 product. Overall, this process converts the two CO units to an ethynol derivative and proceeds easily even at temperatures lower than room temperature.
Agapie said, “To our knowledge, this is the first example of a well-defined reaction that can take two carbon monoxide molecules and convert them into a metal-free ethynol derivative, a molecule related to ethanol; the fact that we can release the C2 product from the metal is important.”
While the generated ethynol derivative is not useful as a fuel, it represents a step toward being able to generate synthetic multicarbon fuels from carbon dioxide. The researchers are now applying the knowledge gained in this initial study to improve the process. “Ideally, our insight will facilitate the development of practical catalytic systems,” Buss said.
The scientists are also working on a way to cleave the C-O bond using protons instead of silyl electrophiles. “Ultimately, we’d like to use protons from water and electron equivalents derived from sunlight,” Agapie said. “But protons are very reactive, and right now we can’t control that chemistry.”
Conversion chemistries for the production of liquid fuels from oxygenated carbon precursors have already been reported. Using copper electrocatalysts, CO and CO2 can be converted to multicarbon products. The process proceeds under mild conditions, but how it takes place remains a mystery. Where these concepts might go is still not something easy to project.
The Fischer-Tropsch process, now about a century old, has been utilized to convert hydrogen gas (H2) and CO to liquid fuels. However, its mechanism is not well understood either and, in contrast to nature’s photosynthesis, the F-T process requires high pressures (from 1 to 100 times atmospheric pressure) and temperatures (100-300º C), making it less than cost effective.
The Caltech team’s work is welcome. Gathering CO2 from the air and making fuel again, over and over has a great appeal. This might be a benchmark on the way to such an artificial cycle. But for now the natural photosynthesis cycle has the lead. Surprisingly, its a hard one to catch up with.
Comments
1 Comment so far


About a year ago, or perhaps a bit earlier, the Naval Research Laboratory reported the development of a new iron-based catalyst that improves the Fischer-Tropsch process. The Navy proposes to make jet fuel on aircraft carriers using carbonate extracted from seawater, where it is much more concentrated than CO2 in the atmosphere.