Mar
24
A Better Catalyst Than Platinum?
March 24, 2015 | Leave a Comment
The Agency for Science, Technology and Research (A*STAR) has announced an oxide/carbon composite outperforms expensive platinum composites in oxygen chemical reactions for green energy devices. Electrochemical devices are essential for a green energy evolution in which clean alternatives replace carbon-based fuels.
Green energy device market growth requires conversion systems that produce hydrogen from water or rechargeable batteries that can store clean energy in cars. The Singapore-based researchers have developed improved catalysts as electrodes for more efficient and durable green energy devices.
Electrochemical devices such as batteries use chemical reactions to create and store energy. One of the cleanest reactions is the conversion from water into oxygen and hydrogen. Using energy from the sun, water can be converted into those two elements, which then store this solar energy in gaseous form. Burning or reoxidizing hydrogen leads to a chemical reaction that produces water.
For technical applications looking for electrons rather than heat, the conversion from hydrogen and oxygen into water is done in fuel cells, while some rechargeable batteries use chemical reactions based on oxygen to store and release energy. A crucial element for both types of devices is the cathode, which is the electrical contact where these reactions take place.
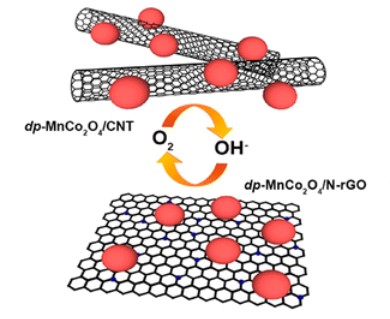
For a well-functioning cathode, the electronic energy levels of the cathode material need to be well matched to the energies required for the oxygen reactions. An ideal material for such reactions is MnCo2O4, a spinel oxide, which has the advantage that its energy states can be fine tuned by adjusting its composition.
The research team, which included Zhaolin Liu and colleagues from the A*STAR Institute of Materials Research and Engineering with colleagues from Nanyang Technological University and the National University of Singapore, combined nanometer-sized crystals of this material with sheets of carbon or carbon nanotubes.
These composites offer several benefits including low cost and high efficiency.
Liu said, “The cost is estimated to be tens of times cheaper than the platinum/carbon composites used at present.” Because platinum is expensive, intensive efforts are being made to find alternative materials for batteries.
The A*STAR researchers fabricated these composites using a scalable chemical synthesis method and studied their performance in oxygen reactions. In these tests, the composites clearly outperformed the platinum-based alternatives. They were more efficient than the platinum-based solutions, with comparable devices in the lab lasting about five times longer, for more than 64 charge-discharge cycles.
While these are still research laboratory results, the first results for full battery prototypes are encouraging, comments Liu. “We envisage a 100-watt rechargeable battery stack in one to two years and a 500-watt one in one to three years.”
The team is off to a great start on what one would hope is a grand success. Much of the technology for hydrogen is held back by the immense barrier of platinum’s cost. And as rare as platinum is, added to its demand would only exacerbate the problem. Lets hope the team’s effort to “harden up” their discovery bears fruit and soon.