Aug
14
Copper Foam Can Make CO2 More Useful
August 14, 2014 | 3 Comments
Brown University scientists have discovered that copper foam could provide a new way of converting excess carbon dioxide into useful industrial chemicals. The catalytic foam is foamy form of copper with vastly different electrochemical properties from catalysts made with smooth copper in reactions involving carbon dioxide.
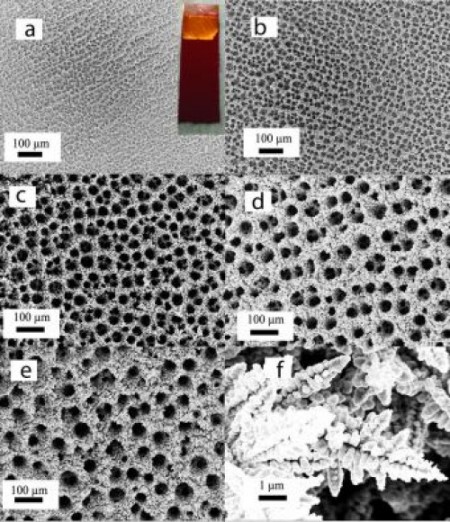
Catalytic Copper Foam at Increasing Magnification. Image Credit: Palmore lab/Brown University. Click image for the largest view.
Oxidizing fuels releases CO2 making its recycling an attractive means to save on fuels and to control and participate in the carbon cycle more responsibly. One approach is to capture CO2 and once sequestered, researchers are looking for ways to make use of it. The problem is that CO2 is an extremely stable molecule, and reducing it to a reactive and useful form isn’t easy.
Tayhas Palmore, professor of engineering and senior author of the new research explains, “Copper has been studied for a long time as an electrocatalyst for CO2 reduction, and it’s the only metal shown to be able to reduce CO2 to useful hydrocarbons. There was some indication that if you roughen the surface of planar copper, it would create more active sites for reactions with CO2.”
Copper foam, which has been developed only in the last few years, provided the surface roughness that Palmore and her colleagues were looking for. The foams are made by depositing copper on a surface in the presence of hydrogen and a strong electric current. Hydrogen bubbles cause the copper to be deposited in an arrangement of sponge-like pores and channels of varying sizes.
Experiments were performed by Sujat Sen and Dan Liu, graduate students in chemistry working in Palmore’s lab at Brown’s School of Engineering to see what happens after depositing copper foams on an electrode and to see what kinds of products would be produced in an electrochemical reaction with CO2 in water.
The experiments showed that the copper foam converted CO2 into formic acid, a compound often used as a feedstock for microbes that produce biofuels, at a much greater efficiency than planar copper. The reaction also produced small amounts of propylene, a useful hydrocarbon that’s never been reported before in reactions involving copper.
Palmore said, “The product distribution was unique and very different from what had been reported with planar electrodes, which was a surprise. We’ve identified another parameter to consider in the electroreduction of CO2. It’s not just the kind of metal that’s responsible for the direction this chemistry goes, but also the architecture of the catalyst.”
Now that it’s clear that architecture matters, Palmore and her colleagues are working to see what happens when that architecture is tweaked. It’s likely, she says, that pores of different depths or diameters will produce different compounds from a CO2 feedstock. Ultimately, it might be possible to tune the copper foam toward a specific desired compound.
Palmore said she’s amazed by the fact that there’s still more to be learned about copper.
“People have studied electrocatalysis with copper for a couple decades now,” she said. “It’s remarkable that we can still make alterations to it that affect what’s produced.”
The work in the study is part of a larger effort by Brown’s Center for the Capture and Conversion of CO2. The Center, funded by the National Science Foundation, is exploring a variety of catalysts that can convert CO2 into usable forms of carbon.
“The goal is to find ways to produce some of the world’s largest-volume chemicals from a sustainable carbon source that the Earth not only has in excess but urgently needs to reduce,” said Palmore, who leads the center. “This is a way for us as scientists to begin thinking of how we produce industrial chemicals in more sustainable ways and control costs at the same time. The cost of commodity chemicals is going nowhere but up as long as production is dependent on fossil fuels.”
CO2 has value that so far, only the plants have been successfully exploiting as a food source. Recycling back some of that value would go far in keeping the fossil fuels in the market longer by recovering some of their cost as well as making second and repeated use of the carbon a part of modern human activity.
Comments
3 Comments so far


Re-usable CO2. This could change everything.
In the words of Arthur C. Clark – “The future is not what it used to be.”
The figure seems miscaptioned. Photos a) through 3) are all the same magnification, according to the scale markers.
Typo: I meant a) through e), not a) through 3).