Mar
11
Progress in Developing the Artificial Leaf
March 11, 2014 | Leave a Comment
There’s progress on hydrogen based fuel from efforts to produce fuels with artificial photosynthesis known commonly as the artificial leaf. A new study by Berkeley Lab researchers at the Joint Center for Artificial Photosynthesis (JCAP) shows that nearly 90 percent of the electrons generated by a hybrid material designed to store solar energy in hydrogen are being stored in the target hydrogen molecules.
Gary Moore, a chemist and principal investigator with Berkeley Lab’s Physical Biosciences Division explains, “Ultimately the renewable energy problem is really a storage problem. Given the intermittent availability of sunlight, we need a way of using the sun all night long. Storing solar energy in the chemical bonds of a fuel also provides the large power densities that are essential to modern transport systems. We’ve shown that our approach of coupling the absorption of visible light with the production of hydrogen in a single material puts photoexcited electrons where we need them to be, stored in chemical bonds.”
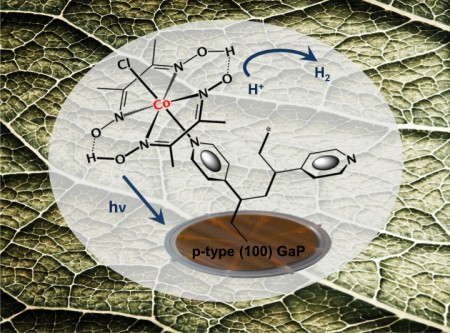
The JCAP research is an efficiency analysis study of a unique photocathode material developed for catalyzing the production of hydrogen fuel from sunlight. The material is a hybrid formed from interfacing the semiconductor gallium phosphide with a molecular hydrogen-producing cobaloxime catalyst. The hybrid material has the potential to address one of the major challenges in the use of artificial photosynthesis to make renewable solar fuels.
Leaves in nature produce energy-dense fuels from nothing more than sunlight, water and carbon dioxide, with no byproducts other than oxygen. Artificial leaves represent an ideal sustainable energy alternative to fossil fuels. But, realizing this artificial photosynthesis ideal will require a number of technological breakthroughs including high performance photocathodes that can catalyze fuel production from sunlight alone.
Last year, Moore and his team took an important step towards the photocathode goal with their gallium phosphide/cobaloxime hybrid. Gallium phosphide is an absorber of visible light, which enables it to produce significantly higher photocurrents than semiconductors that only absorb ultraviolet light. The cobaloxime catalyst is also Earth-abundant, meaning it is a relatively inexpensive replacement for the highly expensive precious metal catalysts, such as platinum, currently used in many solar-fuel generator prototypes.
Moore said, “The novelty of our approach is the use of molecular catalytic components interfaced with visible-light absorbing semiconductors. This creates opportunities to use discrete three-dimensional environments for directly photoactivating the multi-electron and multi-proton chemistry associated with the production of hydrogen and other fuels.”
The efficiency analysis performed by Moore and his colleagues also confirmed that the light-absorber component of their photocathode is a major bottleneck to obtaining higher current densities. Their results showed that of the total number of solar photons striking the hybrid-semiconductor surface, measured over the entire wavelength range of the solar spectrum (from 200 to 4,000 nanometers) only 1.5-percent gave rise to produce a photocurrent.
“This tells us that the use of light absorbers with improved spectral coverage of the sun is a good start to achieving further performance gains, but it is likely we will also have to develop faster and more efficient catalysts as well as new attachment chemistries. Our modular assembly method provides a viable strategy to testing promising combinations of new materials,” Moore says.
“Efficiency is not the only consideration that should go into evaluating materials for applications in solar-fuel generator technologies. Along with the durability and feasible scalability of components, the selectivity of photoactivating a targeted reaction is also critical. This is where molecular approaches offer significant opportunities, especially in catalyzing complex chemical transformations such as the reduction of carbon dioxide.”
In more plain terms what Moore offers is that as well as converting solar energy efficiently into hydrogen there is the need to build an artificial leaf that would build and store a fuel type chemical, perhaps one that would be based on carbon dioxide raw material.
The hydrogen economy idea has been around for decades. Its been stalled by first be coming up with free hydrogen and second storing the smallest atom in H or the smallest molecule in H2. For the hydrogen economy folks the purists still hope for using simply hydrogen gas. Chemists and physicists have invested lifetimes in the effort.
Its refreshing to see that now the natural history of this planet in this solar system over millions of years of biology using hydrogen bonded to carbon is gaining more traction in the science of producing inexhaustible fuel resources.