Dec
18
Breaking Through The Lithium Ion Battery Problems
December 18, 2013 | Leave a Comment
We’ve heard and many have experienced the lithium ion battery problems of overheating and sometimes breaking out into fires.
Over the course of several battery charge/discharge cycles, particularly when the battery is cycled at a fast rate, microscopic fibers of lithium, called “dendrites,” sprout from the surface of the lithium electrode and spread like kudzu across the electrolyte until they reach the other electrode. Then an electrical current passing through these dendrites can short-circuit the battery, causing it to rapidly overheat and in some instances catch fire.
Efforts to solve the problem by curtailing dendrite growth have met with limited success, but now because as the Berkley Lab folks can now show, perhaps they’ve just been scratching the surface of the problem.
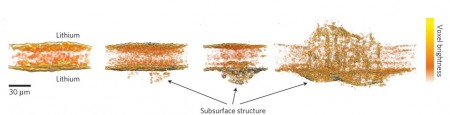
The Berkley Lab team has discovered that during the early stages of development, the bulk of dendrite material lies below the surface of the lithium electrode, underneath the electrode/electrolyte interface.
The team used X-ray microtomography at Berkeley Lab’s Advanced Light Source (ALS) and observed the seeds of dendrites forming in lithium anodes and growing out into a polymer electrolyte during cycling. It was not until the advanced stages of development that the bulk of dendrite material was in the electrolyte. Balsara and his colleagues suspect that non-conductive contaminants in the lithium anode trigger dendrite nucleation.
Balsara explains saying, “Contrary to conventional wisdom, it seems that preventing dendrite formation in polymer electrolytes depends on inhibiting the formation of subsurface dendritic structures in the lithium electrode. In showing that dendrites are not simple protrusions emanating from the lithium electrode surface and that subsurface non-conductive contaminants might be the source of dendritic structures, our results provide a clear prescription for the path forward to enabling the widespread use of lithium anodes.”
The tremendous capacity of lithium and the metal’s remarkable ability to move lithium ions and electrodes in and out of an electrode as it cycles through charge/discharge make it an ideal anode material. Until now, researchers have studied the dendrite problem using various forms of electron microscopy. This is the first study to employ microtomography using monochromatic beams of high energy or “hard” X-rays, ranging from 22 to 25 keV, at ALS beamline 8.3.2. This technique allows non-destructive three-dimensional imaging of solid objects at a resolution of approximately one micron.
Katherine Harry, a member of Balsara’s research group and the lead author of the paper explores the results saying, “We observed crystalline contaminants in the lithium anode that appeared at the base of every dendrite as a bright speck. The lithium foils we used in this study contained a number of elements other than lithium with the most abundant being nitrogen. We can’t say definitively that these contaminants are responsible for dendrite nucleation but we plan to address this issue by conducting in situ X-ray microtomography.”
Balsara and his team also plan further study of the role played by the electrolyte in dendrite growth, and they have begun to investigate ways to eliminate non-conductive impurities from lithium anodes.
Balsara is also a professor of chemical engineering at the University of California (UC) Berkeley and the corresponding author of a paper describing the research in Nature Materials titled “Detection of Subsurface Structures Underneath Dendrites Formed on Cycled Lithium Metal Electrodes.” The lead author is Katherine Harry with co-authors Daniel Hallinan, Dilworth Parkinson and, Alastair MacDowell.
The research is a welcome breakthrough that should have impacts soon. Manufacturers have come some way in better manufacturing, few laptops are roasting thighs now, but notably lithium ion batteries have still caught fire. Of late were the problems on board the Boeing Dreamliner. For Boeing, the investigators, the battery manufacturer and passengers and crew the Berkley research is more than just welcome.
The technological improvements will be “slow” in coming, but the lithium ion batteries of the future look to be much better than the quite impressive ones we have now. The added technology might not add much in cost at commercial scale, but the research suggests a much longer lasting and much safer lithium ion battery.