Dec
17
New Sunlight Powered Hydrogen Production From Water Idea
December 17, 2013 | Leave a Comment
University of Houston (UH) scientists have found a catalyst that can quickly generate hydrogen from water using sunlight’s solar power. The new technique involves the use of cobalt oxide nanoparticles to split water into hydrogen and oxygen.
The research paper has just been published online in Nature Nanotechnology, behind a paywall.
Professor Jiming Bao, lead author of the paper and an assistant professor in the Department of Electrical and Computer Engineering with appointments in materials engineering and the Department of Chemistry at UH, said the research discovered a new photocatalyst and demonstrated the potential of nanotechnology in engineering a material’s property. However, more work is yet to be done.
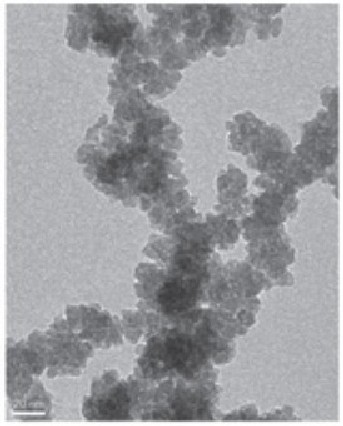
Bao explores the background explaining photocatalytic water-splitting experiments have been tried since the 1970s, but the Houston effort was the first to use cobalt oxide and the first to use neutral water under visible light at a high-energy conversion efficiency without co-catalysts or sacrificial chemicals. The project involved researchers from UH, along with those from Sam Houston State University, the Chinese Academy of Sciences, Texas State University, Carl Zeiss Microscopy LLC, and Sichuan University.
The research team prepared the nanoparticles in two ways, using femtosecond laser ablation and through mechanical ball milling. Despite some differences, Bao said both worked equally well.
During the experimentation different sources of light were used, ranging from a laser to white light simulating the solar spectrum. Bao said he would expect the reaction to work equally well using natural sunlight.
Once the nanoparticles are added and light applied, the water separates into hydrogen and oxygen almost immediately, producing twice as much hydrogen as oxygen, as expected from the 2:1 hydrogen to oxygen ratio in H2O water molecules.
This is quite interesting news, yet there remains a leavening. The experimental result has the potential as a source of renewable fuel, but at a solar-to-hydrogen efficiency rate of around 5 percent, the conversion rate is still too low to be commercially a viable process.
Bao suggested a more feasible efficiency rate would be about 10 percent, meaning that 10 percent of the incident solar energy will be converted to hydrogen chemical energy by the process.
There are other issues remaining to resolve as well. The list includes reducing costs and extending the lifespan of cobalt oxide nanoparticles, which the researchers found became deactivated after about an hour of reaction.
Bao recognizes the issues clearly saying, “It degrades too quickly.”
The work is being supported by the Welch Foundation and will lead to future research, Bao said. The research is needed for understanding the question of why cobalt oxide nanoparticles have such a short lifespan, and questions involving chemical and electronic properties of the material.
As these matters are explored the answers may well lead to a very enticing hydrogen production module on the market someday. It’s a great first step into a new unknown.