Jul
3
A New Far Cheaper Hydrogen Production Catalyst
July 3, 2013 | 2 Comments
A catalyst discovery at the University of Wisconsin-Madison could be a significant advance in the quest to create a “hydrogen economy” that would use hydrogen to store and transfer energy. Catalysts speed up chemical reactions without themselves being consumed.
Mark Lukowski, a Ph.D. student working with associate professor Song Jin in the UW-Madison chemistry department explains the material under study, molybdenum disulfide, commonly used as a lubricant, contains two common elements. “Most people have tried to reduce the cost of the catalyst by making small particles that use less platinum, but here we got rid of the platinum altogether and still got reasonably high performance,” he said.
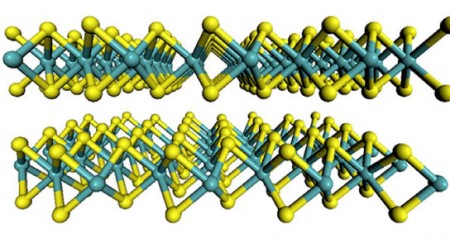
Molybdenum Disulfide in Two Dimensions. The flat-sheet structure of the material molybdenum disulfide with molybdenum atoms are shown in teal and the sulfur atoms in yellow. Click image for the largest view.
The research group has produced milligram quantities of the catalyst, “but in principle you could scale this up,” says Lukowski. “Molybdenum disulfide is a commercially available product. To control purity and structure, we go through the trouble of synthesizing it from the bottom up, but you could buy it today.”
Theoretically, hydrogen is the ultimate non-carbon, non-polluting fuel for storing intermittent energy from the wind or sun. When burned for energy, hydrogen produces water but no carbon dioxide. Practically speaking, producing hydrogen from water, and then storing and using the gas, has proven difficult.
The important point is the new catalyst avoids the rare, expensive metal platinum that is normally required for this reaction.
To make the new material, Lukowski and Jin deposit nanostructures of molybdenum disulfide on a disk of graphite and then apply a lithium treatment to create a different structure with different properties.
Just as carbon can form diamond for jewelry and graphite for writing, molybdenum disulfide can be a semiconductor or a metallic phase, depending on structure. When the compound is grown on the graphite, it is a semiconductor, but it becomes metallic after the lithium treatment. Lukowski and Jin discovered that the metallic phase has far greater catalytic properties.
Lukowski explains, “Like graphite, which is made up of a stack of sheets that easily separate, molybdenum disulfide is made up of individual sheets that can come apart, and previous studies have shown that the catalytically active sites are located along the edges of the sheets.”
“The lithium treatment both causes the semiconducting-to-metallic phase change and separates the sheets, creating more edges. We have taken away the limitation from molybdenum disulfide and made the active sites both more pervasive and more reactive,” Lukowski said.
Jin notes the experiment, supported by the U.S. Department of Energy’s Basic Energy Sciences program, is a proof of concept for a new approach for improving these catalysts.
Jin takes up the explanation with, “Even though the efficiency of producing hydrogen has been greatly improved, it is still not as good as what platinum can achieve. The next steps include finding ways to further improve the performance by optimizing all aspects of the process and exploring related compounds. There are many hurdles to achieving a hydrogen economy, but the advantages in efficiency and pollution reduction are so significant that we must push ahead.”
Jin is up to speed on the platinum issue for a catalyst saying as technological advances put further strain on the supply of platinum and other rare elements, using common elements is a major advantage. “The elements we use are cheap and abundant in earth’s crust, and the raw material is already commercially available at low cost. Building on this discovery and new understanding, we would like to further improve these materials to achieve the efficient production of hydrogen without using precious metals.” He said.
Is the hydrogen production next great step finally here? Lukowski might well be right, the elements are certainly low cost. Just how the team’s production of the working catalyst will scale isn’t yet known. The production process as explained doesn’t imply huge expense or inventing new processes.
This does look very good indeed; perhaps good enough and further research may surprise us. Now if one could keep the hydrogen stored and used with little loss instead of leaking it ultimately into outer space.
Comments
2 Comments so far


Catalysts are alright but what about energy input? It would be useful only if you can use freely available solar energy or plentiful nuclear heat.
I definitely wanted to make a quick remark to express gratitude
to you for those stunning tactics you are sharing on
this website. My particularly long internet lookup has at
the end been rewarded with extremely good information to go over
with my close friends. I ‘d tell you that we website visitors are very lucky to live
in a really good site with so many marvelous
people with interesting methods. I feel really fortunate to have discovered the web page and look forward to really more fabulous
minutes reading here. Thanks a lot once more for all the details.