May
30
Stanford University scientists have developed an advanced zinc-air battery with higher catalytic activity and durability. The new battery design also avoids using the expensive platinum and iridium catalysts. The two improvements could lead to the development of a low-cost alternative to today’s widely used conventional lithium-ion batteries.
Hongjie Dai, a professor of chemistry at Stanford and lead author of the study opens with, “There have been increasing demands for high-performance, inexpensive and safe batteries for portable electronics, electric vehicles and other energy storage applications. Metal-air batteries offer a possible low-cost solution.”
According to Dai, most research attention has focused on lithium-ion batteries, despite their limited energy density (energy stored per unit of volume), high cost and safety problems. “With ample supply of oxygen from the atmosphere, metal-air batteries have drastically higher theoretical energy density than either traditional aqueous batteries or lithium-ion batteries,” he said. “Among them, zinc-air is technically and economically the most viable option.”
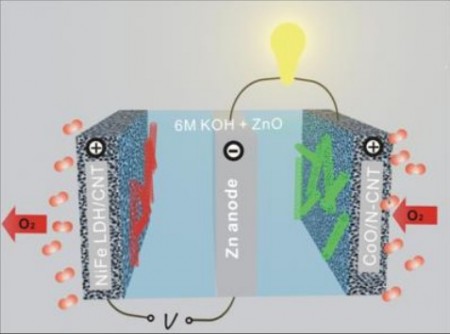
Simply explained zinc-air batteries combine atmospheric oxygen and zinc metal in a liquid alkaline electrolyte to generate electricity with a byproduct of zinc oxide. When the process is reversed during recharging, the oxygen and zinc metal are regenerated.
Dai continues, “Zinc-air batteries are attractive because of the abundance and low cost of zinc metal, as well as the non-flammable nature of aqueous electrolytes, which make the batteries inherently safe to operate. Primary (non-rechargeable) zinc-air batteries have been commercialized for medical and telecommunication applications with limited power density. However, it remains a grand challenge to develop electrically rechargeable batteries, with the stumbling blocks being the lack of efficient and robust air catalysts, as well as the limited cycle life of the zinc electrodes.”
Dai explains the technical issue that active and durable electrocatalysts on the air electrode are required to catalyze the oxygen-reduction reaction during discharge and the oxygen-evolution reaction during recharge. In zinc-air batteries, both catalytic reactions are sluggish.
The improvement his group has developed is a number of high-performance electrocatalysts made with non-precious metal oxide or nanocrystals hybridized with carbon nanotubes. These catalysts produced higher catalytic activity and durability in alkaline electrolytes than catalysts made with platinum and other precious metals.
Alone that is significant news.
Dai said, “We found that similar catalysts greatly boosted the performance of zinc-air batteries for both primary and rechargeable types. A combination of a cobalt-oxide hybrid air catalyst for oxygen reduction and a nickel-iron hydroxide hybrid air catalyst for oxygen evolution resulted in a record high-energy efficiency for a zinc-air battery, with a high specific energy density more than twice that of lithium-ion technology.”
This is a huge incentive improvement over using platinum or iridium in further development.
The novel battery also demonstrated good reversibility and stability over long charge and discharge cycles over several weeks.
Dai wound up the press release quotes with, “This work could be an important step toward developing practical rechargeable zinc-air batteries, even though other challenges relating to the zinc electrode and electrolyte remain to be solved.”
The choice of ‘could” on the potential is conservative and cautionary. The issues on the zinc side and the electrolyte are matters that “could” be found quite soon. Still the air, zinc and electrolyte all have to work well together over perhaps thousands of charge and recharge cycles. The mission isn’t done quite yet.
The professor’s work does take the research into a new vista of possibilities. The chance to avoid the platinum or iridium expense is extremely hopeful. Dai leads a good-sized team including Yanguang Li, Ming Gong, Yongye Liang, Ju Feng, Ji-Eun Kim, Hailiang Wang, Guosong Hong, and Bo Zhang. Lots of potential there.
Go Stanford!
Comments
1 Comment so far


Something I’ve been wondering about with metal-air batteries (any metal-air battery), and I haven’t seen it addressed anywhere else.
During discharge, they consume oxygen from the air. That’s OK, gas cars do it every day.
But during recharge, they release oxygen into the air. How much oxygen are we talking about? Is it enough to be hazardous? Are you risking burning down your house by recharging your metal-air car in a closed garage?