Jan
30
Next – Bugs That Eat Electricity and Make Fuel
January 30, 2013 | 1 Comment
University of Minnesota scientists have developed a way to grow iron-oxidizing bacteria using electricity instead of iron. The new development opens the possibility for further study that could lead to developing organisms that could one day be used to turn electricity into fuel. All civilization would need then is an abundance of electrical generating capacity.
The method is called electrochemical cultivation where electricity supplies the specialized bacteria with a steady supply of electrons that the bacteria use to respire, or “breathe.” This development opens the possibility that one day electricity generated from renewable sources like wind or solar could be funneled to iron oxidizing bacteria that combine it with carbon dioxide to create biofuels, capturing the energy as a useful, storable substance. It’s a whole new take on storage of intermittent power sources.
Daniel Bond of the BioTechnology Institute at the University of Minnesota – Twin Cities, who co-authored the paper with Zarath Summers and Jeffrey Gralnick said, “It’s a new way to cultivate a microorganism that’s been very difficult to study. But the fact that these organisms can synthesize everything they need using only electricity makes us very interested in their abilities.”
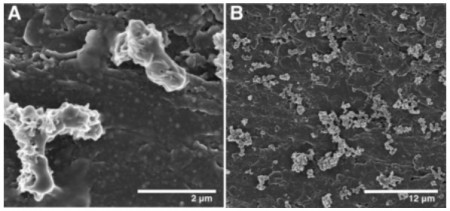
A little background from the press release – to “breathe,” iron oxidizers take electrons off of dissolved iron, called Fe(II) – a process that produces copious amounts of rust, called Fe(III). Iron-oxidizing bacteria are found around the world; almost anywhere an aerobic environment (with plenty of oxygen) meets an anaerobic environment (which lacks oxygen). They play a big role in the global cycling of iron and contribute to the corrosion of steel pipelines, bridges, piers, and ships, but their lifestyle is at the interface of two very different habitats and the accumulation of cell-trapping Fe(III) makes iron oxidizers difficult to grow and study in the lab.
Scientists have been thinking these bacteria must carry out the iron oxidation step on their surfaces. If that’s true, Bond reasons, the outsides of the organisms should be covered with proteins that interact with Fe(II), so you should be able to provide a stream of pure electrons to the outsides of the bacteria and get them to grow.
So Bond and his colleagues added the marine iron oxidizer Mariprofundus ferrooxydans PV-1, along with some nutrient medium, to an electrode carefully tuned to provide electrons at the same energy level, or potential, as Fe(II) would provide. The idea, says Bond, was to “fool the bacteria into thinking they’re at the world’s best buffet of Fe(II) atoms.”
The experiment worked.
The bacteria multiplied and formed a film on the electrode, Bond says, and eventually they were able to grow M. ferrooxydans with no iron in the medium, proof that the bacteria were living off the electrons they absorbed from the electrode to capture carbon dioxide and replicate. And since the electron donor is a solid surface, say the authors, it’s pretty likely that the bacterial electron-harvesting machinery is exposed on the outer membrane of the cell.
Now here is the important part – it is this means of capturing of carbon dioxide that could enable electrochemical cultivation to create biofuels or other useful products one day, Bond said.
Bond explains the potential with, “Bacteria are experts at the capture of carbon dioxide. They build cells and compounds” with the carbon, he says. They might one day be exploited as microscopic energy packagers: bacteria like M. ferrooxydans could capture electricity from an electrode, combine it with carbon dioxide, and package it as a carbon-rich compound we could use as fuel. This would take the energy in electricity, which is ephemeral, and convert it into a tangible product that could be stored in a tank.
However, Bond cautions, “But that kind of work is a long way off. If there are 100 steps to making this work – this is step one.”
All well and good, Professor. But, you and your team found step one and have shown that it works. Congratulations!
Comments
1 Comment so far


As any electrochemist knows, electrons are by far the cheapest reactant you can use!
JP Straley