Feb
13
Building a Better Hydrogen Fuel Catalyst
February 13, 2012 | 6 Comments
In a stunning statement, University of California, Berkeley (UCB), chemists using an engineered catalyst in single molecule form that when dropped into acidic water and even seawater, the catalyst molecules generated hydrogen for several days without letup.
Quote, “When lots of these single-molecule catalysts were dumped into acidic water and even seawater, they generated hydrogen for several days without letup.” Your humble writer is contacting the press release author and asking that the statement be confirmed or corrected. If the statement is factually correct, then the UCB chemists have cracked open a major opportunity to get a source of free hydrogen without energy input – creating a true threshold for an energy production revolution. Lets hope the report is correct.
*Update Feb 15, 2012:
Hi, Brian. There is, of course, an energy input – electricity. They are trying to generate that electricity from solar. I should have spelled that out and not assumed it to be a given.–Bob
We’ll ask about efficiency as they make progress.*
Meanwhile, getting to that point is research led by Christopher Chang, associate professor of chemistry and Howard Hughes Medical Institute Investigator at UCB. The study paper was published last week the journal Science, showing how to construct a catalyst composed only of edges and demonstrate that it can catalyze the production of hydrogen from water as readily as the edges and defects in regular catalysts.
Chang said, “This is a conceptual advance in the way we think about generating hydrogen, a clean burning fuel, from water, a sustainable source. Our new catalyst is just first generation, but the research gives us and the community a path forward to thinking about how to increase the density of functional active sites so that molecules and materials can be more effective catalysts.”
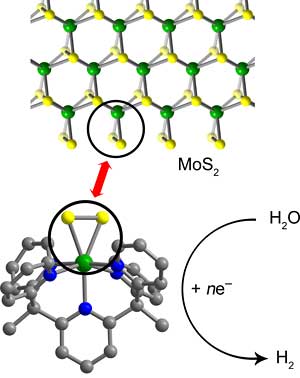
Chang and the UCB research team are working with a common catalyst, molybdenite, which is less expensive than platinum. Molybdenite is composed of molybdenum and sulfur (MoS2) and is getting increasing interest as a fuel cell catalyst. When built up to an optimum shape the material catalyzes reactions like the splitting of water into hydrogen and oxygen only at the edges, where triangles of molybdenum and two sulfur atoms stick out like pennants to react with the reactants.
Change explains, “These edge sites look like little MoS2 triangles, and the triangular area does the business.”
Customarily catalysts are usually metals that speed up chemical reactions. They’re used across the chemical industry, petroleum industry and as close to each of us as the catalytic converter in automobile exhaust. The big goal in catalytic conversion is the fuel cell, to extract the energy of hydrogen into electricity.
The UCB team is seeking to find ways to expose as much as possible of the catalyst material surface where the chemistry can be assisted in the reaction. The engineering then would greatly reduce the amount of catalyst needed to get the reaction accomplished.
Chang and his colleagues created a small carbon framework using complex organic synthesis techniques to hold the MoS2 triangle so that “every molecule has a discrete edge site that is a catalytically active unit.” Those are what are thought to have been dropped into to the acidic and sea water.
The future goal is maximizing the number of catalytic sites for a given volume and boosting ultimate efficiency. Chang hopes to assemble billions of these molecules on a thin, ridged wafer.
Looking ahead Chang said, “There are many other types of materials out there for which people might want to generate edge-site fragments rather than use a bulk material with just a few edge or defect sites. With hydrogen being touted as a clean burning fuel that generates no CO2, creating cheaper and better catalysts has become a big and important field now. The main push is toward more earth-abundant materials than the rare metals like platinum.”
The UCB team members working with Chang are Jeffrey R. Long, UC Berkeley professor of chemistry and faculty scientist at Lawrence Berkeley National Laboratory, professor of chemistry Marcin Majda, and post-doctoral fellows Hemamala I. Karunadasa, Elizabeth Montalvo and Yujie Sun.
It will be quite an interesting development if the UCB team has established threshold of producing hydrogen without an energy input. One would expect intense efforts to get practical systems at scale as soon as possible at economical pricing. A low cost, long lasting catalyst making cheap hydrogen would be a sea change in energy economics.
The research abstract hints and the press release is quite bold. One sure hopes its not an error or misinterpretation.
Comments
6 Comments so far


I would be very careful. You don’t get something for nothing in chemistry. I could offer quicklime as an example. I buy a bag of the stuff and dump in a tub of water. The exothermic reaction I use to heat my house. What is not revealed is the huge chunk of energy that went into originally slaking the chalk into quicklime to begin with.
Just sayin’.
Burn it directly? Why submit to the energy conversion limits of the Carnot cycle? Why fiddle about with the hassle of storing H2 gas?
But if you combine with syngas, can make easily storable fuels suitable for the existing fleet of vehicles. Industrial H now comes from nat gas, so this is an excellent advance.
And how about fuel cells? That’s the hot ticket for his technology.
the most important part was left out of your summary
“When lots of these single-molecule catalysts were dumped into acidic water and even seawater ***and electrodes inserted to provide electrical current,*** they generated hydrogen for several days without letup.”
Leaving out the electrical current makes one think this process is free. Was that wilful?
CC
Hi, Brian. There is, of course, an energy input – electricity. They are trying to generate that electricity from solar. I should have spelled that out and not assumed it to be a given.–Bob
Bob Sanders
Manager, Science communications
UC Berkeley Media Relations
hydrogen is the best fuel that we could ever have since it is non polluting. .
Hydrogen fuel is of course the fuel of the future, it is non-polluting and renewable.