Feb
8
The Best Lithium Air Batteries Get a 33% Boost
February 8, 2012 | Leave a Comment
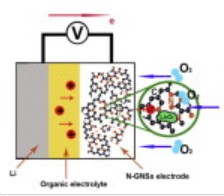
The best lithium air lab research batteries had cathodes built with graphene nanosheet materials. Back in August of 2011 scientists at the Nanomaterials and Energy Group at the University of Western Ontario (UWO), Canada, reported the development of graphene nanosheet cathode (GNS) materials for non-aqueous lithium-oxygen (Li-air) batteries showing a capacity of 8,705.9 mAh g-1 – the highest capacity of any carbon-based materials in lithium-oxygen batteries reported to that time.
This week the UWO scientists report that using nitrogen-doped graphene nanosheets (N-GNSs) as cathode materials significantly increases the performance of a non-aqueous lithium-oxygen battery by about 33% more than the high-performance pristine graphene nanosheets announced in August.
The paper has been published in the journal Electrochemistry Communications.
The research team led by Professor Xueliang (Andy) Sun found that their nitrogen-doped graphene nanosheet cathode materials delivered a discharge capacity of up to 11,660 mAh g-1 compared to the pristine graphene nanosheet capacity of up to 8,706 mAh g-1.
The team had been following recent studies where nitrogen doping was applied to carbon powder and nanotubes. Those research efforts showed higher discharge capacities. The UWO team then set out to try nitrogen doping on carbon nanosheets by testing for electrocatalytic activity of N-GNSs for oxygen reduction in the non-aqueous electrolyte. The nitrogen doping for oxygen reduction result is 2.5 times that of the pristine graphene nanosheet. The team is attributing the excellent electrochemical performance of N-GNSs to the defects and functional groups as active sites introduced by the nitrogen doping.
The team is pointing out the finding not only shows that N-GNSs are promising electrode materials, but also gives a rational direction to modify other carbon materials for application in lithium-oxygen batteries.
The hard numbers reported in the study show the initial discharge capacity of the GNS electrode was 8,530 mAh g-1 at a current density of 75 mA g-1, while the N-GNS electrode delivered 11,660 mAh g-1 for N-GNSs an increase about 37% higher.
Keep in mind these are lab research batteries and other questions are coming up. Another worthwhile consideration is as the current densities increased, the discharge capacities of both samples decreased. At a current density doubled to 150 mA g-1 discharge capacities were 5,333 mAh g-1 for GNS and 6,640 mAh g-1 for N-GNS. At 300 mA g-1 capacities went to 3,090 mAh g-1 for GNS and 3,960 mAh g-1 N-GNS. These results are pretty much linear with no big surprises.
Nitrogen isn’t especially expensive, but graphene nano sheets aren’t a common item yet. In fact graphite is usually mined rather than made from petroleum. Thus for now the new cathodes are anything but cheap. But mass markets and scaling up have amazing effects on pricing product components.
Meanwhile . . . down in the U.S. the IBM effort has been working on air batteries too. One or the other of these or perhaps others are going to find a practical and affordable way to store very high capacities of electricity one day. Things have come a long way from carbon dry cells and lead acid.
Maybe the Canadians up at Western can provoke a bit of news out of IBM. Air battery news isn’t coming at raging speed, but the bits we’re getting are encouraging.