Dec
5
Solar Power By Biomimetic Antenna
December 5, 2011 | Leave a Comment
Silicon and printed dye photovoltaic may have a new competitor soon.
At Washington University in St. Louis’s Photosynthetic Antenna Research Center (PARC) one scientific team has just succeeded in making a crucial photosystem component – a light-harvesting antenna – from scratch. The new antenna is modeled on the chlorosome found in green bacteria.
We may not be stuck with just one means to harvest solar energy. The solar cell is only 70 years old and came from a new understanding of semiconductors, materials that can use light energy to create mobile electrons and an electrical current. Comparatively they are quite efficient, yet they have almost nothing to do with the biological photosynthesis in plants that use light energy to push electrons across a membrane and ultimately create sugars and other organic molecules.
Since 1941 no one had the depth of understanding of those complex assemblages of proteins and pigments well enough to exploit their secrets for the design of solar cells.
That’s over now.
At (PARC) scientists are exploring native biological photosystems, building hybrids that combine natural and synthetic parts, and building fully synthetic analogs of natural systems. Now they have a light-harvesting antenna described in a recent issue of New Journal of Chemistry.
Chlorosomes are giant assemblies of pigment molecules. Perhaps Nature’s most spectacular light-harvesting antennae, they allow green bacteria to photosynthesize even in the dim light in ocean deeps.
Dewey Holten, PhD, professor of chemistry in Arts & Sciences, and collaborator Christine Kirmaier, PhD, research professor of chemistry are part of a team that is trying to make synthetic chlorosomes. Holten and Kirmaier use ultra-fast laser spectroscopy and other analytic techniques to follow the rapid-fire energy transfers in photosynthesis.
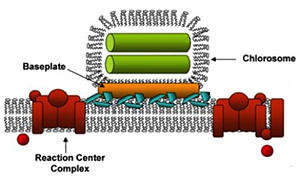
The PARC article explains chlorosomes as biological systems that capture the energy in sunlight and convert it to the energy of chemical bonds. While they come in many varieties, but they all have two basic parts: the light harvesting complexes, or antennae, and the reaction center complexes.
The activity of an antenna consists of many pigment molecules that absorb photons and pass the excitation energy to the reaction centers.
In the reaction centers, the excitation energy sets off a chain of reactions that create ATP, a molecule often called the energy currency of the cell because the energy stored ATP powers most cellular work. Cellular organelles selectively break those bonds in ATP molecules when they need energy hits for cellular work.
The PARC folks are intuitive enough to look at green bacteria, which live in the lower layers of ponds, lakes and marine environments, and in the surface layers of sediments, and have evolved large and efficient light-harvesting antennae very different from those found in plants bathing in sunlight on Earth’s surface.
Green bacteria offers a super antennae consisting of highly organized three-dimensional systems of as many as 250,000 pigment molecules that absorb light and funnel the light energy through a pigment/protein complex called a baseplate to a reaction center, where it triggers chemical reactions that ultimately produce the desired ATP.
In plants and algae (and in the baseplate in the green bacteria) photo pigments are bound to protein scaffolds, which space and orient the pigment molecules in such a way that energy is efficiently transferred between them. But chlorosomes don’t have a protein scaffold – instead the pigment molecules self -assemble into a structure that supports the rapid migration of excitation energy.
That’s intriguing because it suggests chlorosome mimics might be easier to incorporate in the design of solar devices than biomimetics that are made of proteins as well as pigments.
The PARC team’s goal was to see whether synthesized pigment molecules could be induced to self-assemble – even though the process by which the pigments align and bond is not well understood.
Holten explains, “The structure of the pigment assemblies in chlorosomes is the subject of intense debate, and there are several competing models for it.” To design a pigment for a photosynthetic organism a chemist first builds one of three molecular frameworks. All three are macrocycles, or giant rings: porphyrin, chlorin and bacteriochlorin. “One of the members of our team, Jon Lindsey can synthesize analogs of all three pigment types from scratch,” said Holten.
With that in mind the team wanted to study many variations of a pigment molecule to see what favored and what blocked assembly and Lindsey had also developed the means to synthesize chlorins, the basis for the pigments found in the chlorosomes of green bacteria. The chlorins push the absorption to the red end of the visible spectrum, an area of the spectrum scientists would like to be able to harvest for energy.
Doctoral student Olga Mass and coworkers in Lindsey’s lab synthesized 30 different chlorins, systematically adding or removing chemical groups thought to be important for self-assembly but also attaching peripheral chemical groups that take up space and might make it harder for the molecules to stack or that shift around the distributions of electrons so that the molecules might stack more easily.
The powdered pigments were shipped Holten’s lab at WUSTL and to David Bocian’s lab at the University of California at Riverside.
The two labs made up green-tinctured solutions of each of the 30 molecules in small test tubes and then poked and prodded the solutions by means of analytical techniques to see whether the pigment had aggregated and, if so, how much had formed the assemblies. Holten’s lab studied their absorption of light and their fluorescence (which indicated the presence of monomers, since assemblies don’t normally fluoresce) and Bocian’s lab studied their vibrational properties, which are determined by the network of bonds in the molecule or pigment aggregate as a whole.
In one crucial test Joseph Springer at Holten’s lab, compared the absorption spectrum of a pigment in a polar solvent that would prevent it from self-assembling to the spectrum of the pigment in a nonpolar solvent that would allow the molecules to interact with one another and form assemblies.
“You can see them aggregate. A pigment that is totally in solution is clear, but colored a brilliant green. When it aggregates, the solution becomes a duller green and you can see tiny flecks in the liquid,” Springer said.
The absorption spectra indicated that some pigments formed extensive assemblies and that the steric and electronic properties of the molecules predicted the degree to which they would assemble.
The PARC scientists have already taken the next step toward a practical solar device. Along with Pratim Biswas, PhD, the Lucy and Stanley Lopata Professor and chair of the Department of Energy, Environmental & Chemical Engineering the team has demonstrated getting the pigments to self-assemble on surfaces, which is the next step in using them to design solar devices, explained Holten.
Holten cautions, “We’re not trying to make a more efficient solar cell in the next six months. Our goal instead is to develop fundamental understanding so that we can enable the next generation of more efficient solar powered devices.”
There is a very long way to go, and modeling from biology isn’t always a path to success. But knowledge and understanding of biology is growing exponentially. The research here offers a depth of understanding that will have an impact on further engineering efforts.
Man’s ability to grasp and understand vastly speeds up the time compared to what nature has needed to build complex organisms and systems. This research is another example of basic research opening new doors for technology change.