Apr
27
A Low Cost Fuel Cell Catalyst Design
April 27, 2011 | 1 Comment
Maybe this is it, or so close that fuel cells might get some market traction at consumer prices. In a paper published Friday April 22, 2011 in Science, Los Alamos researchers describe the use of a platinum-free catalyst in the cathode of a hydrogen fuel cell. Eliminating platinum the precious metal more expensive than gold, would solve a significant economic challenge that has held up widespread use of large-scale hydrogen fuel cell systems.
Los Alamos researchers Gang Wu, Christina Johnston, and Piotr Zelenay, joined by researcher Karren More of the Oak Ridge National Laboratory, developing a polymer-electrolyte hydrogen fuel cell to convert hydrogen and oxygen into electricity, avoiding the use of expensive platinum. Environmentally friendly fuel cells might replace current power sources in everything from personal data devices to automobiles, if the hydrogen supply can be worked out at low cost.
The team of colleagues developed non-precious-metal catalysts for the part of the fuel cell that reacts with oxygen. The catalyst uses carbon, partially derived from polyaniline in a high-temperature process, and inexpensive iron and cobalt instead of platinum. The result is high power output, good efficiency, and promising longevity. The researchers found that fuel cells containing the carbon-iron-cobalt polymer catalyst (CICP) synthesized by Wu not only generated electrical currents comparable to the output of precious-metal-catalyst fuel cells, but held up favorably when cycled on and off – a condition that can damage inferior catalysts relatively quickly.
The new CICP catalyst fuel cells effectively completed the conversion of hydrogen and oxygen into water, rather than producing large amounts of undesirable hydrogen peroxide. Inefficient conversion of the fuel, which generates hydrogen peroxide, can reduce power output by up to 50 percent, and also has the potential to destroy the fuel cell’s membranes. The CICP catalysts synthesized at Los Alamos create extremely small amounts of hydrogen peroxide, even when compared with the state-of-the-art platinum-based oxygen-reduction catalysts. From a users point of view this is quite good news because the removal of the hydrogen peroxide can be a hazardous nuisance.
Based on the performance results of the CICP catalyst the researcher team has filed a patent for it.
Facing into the platinum cost problem is deal breaker for fuel cells. Alternatives, especially ones that simplify operation is a great step forward. Zelenay said, “The encouraging point is that we have found a catalyst with a good durability and life cycle relative to platinum-based catalysts. For all intents and purposes, this is a zero-cost catalyst in comparison to platinum, so it directly addresses one of the main barriers to hydrogen fuel cells.”
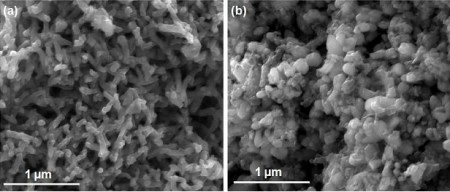
The next step in the team’s research will be to better understand the mechanism underlying the carbon-iron-cobalt catalyst. Micrographic images of portions of the catalyst by researcher Ms. More have provided some insight into how it functions, but further work must be done to confirm theories by the research team. Completing an understanding should lead to improvements in non-precious-metal catalysts, further increasing their efficiency and lifespan.
Zelenay could be effused a bit at the thought of zero-cost, but getting away from thousands or tens of thousands of dollars for the catalyst in fuel cells paints an opportunity that might look like zero in comparison if the CICP catalyst is made in mass volume.
If the team’s fuel cell life expectancy gets far into the tens of thousands of cycles and the cost is low enough the hydrogen fuel production and the storage issues will command much more serious attention with even more investment and innovation. Hydrogen isn’t cheap and still is a devil to hang onto over time.
But a very low cost fuel cell changes the hydrogen economic picture completely. And further work might turn up a very low cost fuel cell that can use methane or light alcohols. It is good to see that the widely read journal Science published the paper. Market traction is something this team needs. For those in the business a full read of the paper is worthwhile.
Comments
1 Comment so far


As Swiper the fox would say, You’re too late! Fuel cells are obsolete, haven’t you heard? Check out http://www.nickelpower.org, see its “news” list. Cold fusion is here, and, with the possibility of very small stuff (laptop or cell phone batteries) it is leaving no room for fuel cells.