Nov
11
Sunlight and Water to Hydrogen and Electricity
November 11, 2009 | 4 Comments
Chemist Daniel Nocera of MIT made news by trying to mimic photosynthesis, and improve on it. The idea seems simple: split water into hydrogen and oxygen with sunlight, and then recombine them which releases the energy in a fuel cell when the power is needed. The goal is to do both these things cheaply. Nocera has come up with a way to split water into its constituent elements that is less expensive than the technology used today.
Nocera’s idea is simple: Split water into hydrogen and oxygen with sunlight. On the other side he says a “new catalyst” for fuel cells is what’s needed, but he’s hopeful that he’ll find it. He predicts success in less than a decade.
Nocera’s paper, Chemistry of Personalized Solar Energy published in Inorganic Chemistry, describes the plan as a solution to the solar energy collection, storage and use using the hydrogen as the storage medium. The paper’s abstract says:
Personalized energy (PE) is a transformative idea that provides a new modality for the planet’s energy future. By providing solar energy to the individual, an energy supply becomes secure and available to people of both legacy and nonlegacy worlds and minimally contributes to an increase in the anthropogenic level of carbon dioxide. Because PE will be possible only if solar energy is available 24 hours a day, 7 days a week, the key enabler for solar PE is an inexpensive storage mechanism. HY (Y = halide or OH−) splitting is a fuel-forming reaction of sufficient energy density for large-scale solar storage, but the reaction relies on chemical transformations that are not understood at the most basic science level. Critical among these are multielectron transfers that are proton-coupled and involve the activation of bonds in energy-poor substrates. The chemistry of these three italicized areas is developed, and from this platform, discovery paths leading to new hydrohalic acid- and water-splitting catalysts are delineated. The latter water-splitting catalyst captures many of the functional elements of photosynthesis. In doing so, a highly manufacturable and inexpensive method for solar PE storage has been discovered.
Nocera is claiming with the paper he has the chemistry worked out with a synthetic solar fuel process that captures many of the elements of photosynthesis outside the plant.
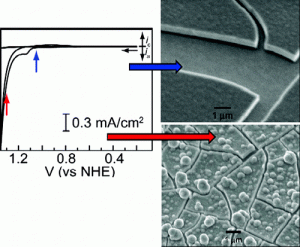
Central to the idea is an innovative oxygen-evolving catalyst that splits water molecules into oxygen and hydrogen that become fuel for producing electricity in a fuel cell.
Nocera points out that low-energy densities characterize most current methods of solar storage, primarily batteries. Thus using batteries presents formidable challenges for large-scale solar personalized energy implementation. Though considerable efforts are currently being devoted to battery development, most advances have little to do with the energy density, but rather they are concerned with the power density (i.e., the rate at which charge can flow in and out of the battery) and the battery life span. Energy densities of batteries are not only low (~0.1−0.5 MJ/kg), but there is little room for improvement because the electron is stored at a metal center of an inorganic network interfacing to an electrolyte.
Nocera understands the paradox of the matter, cheap energy with expensive collection and storage. Going instead to a fuel for storage offers the energy density of liquid fuels (~50 MJ/kg) that’s greater than or equal to 100 times larger than the best of the current methods of solar energy storage.
Nocera’s approach of using the solar energy to produce fuel for a fuel cell could be the key to driving down the investment cost to personalized solar energy. Nocera also sees his solar storage solution as being a great leveler between people of the developed and developing world.Nocera says in his paper, “Because energy use scales with wealth, point-of-use solar energy will put individuals, in the smallest village in the nonlegacy world and in the largest city of the legacy world, on a more level playing field.”
The idea surely has legs. Nocera has the reputation to keep going. But there are some points to keep in mind. Lead acid cells are just not that expensive at about $100 for a kilowatt-hour. A hydrogen fuel cell is going to have to be very low cost. Ultra capacitors, air batteries and other technologies would greatly simplify storage and should reduce the investment. Nocera’s system would need water, and quite interesting is Nocera’s point at 3.1.4 in the paper that Charles River water as it passes by the MIT campus works in the system with a minor 40mV offset in production.
Nocera is on to something with his chemistry. The cobalt oxygen evolving complex (Co-OEC) is the first catalyst to operate in neutral water and hence enables the inexpensive and efficient generation of hydrogen and oxygen from water. Co-OEC is unique because it (1) operates safely with high activity under benign conditions (room temperature and pH 7), (2) is comprised of inexpensive, earth-abundant materials and is easy to manufacture and engineer, (3) is self-healing, (4) is functional in natural water streams and seawater, (5) can form on diverse conducting surfaces of varying geometry and therefore can be easily interfaced with a variety of light-absorbing and charge-separating materials, and (6) may be activated by solar-derived electricity or directly by sunlight mediated by a semiconductor.
Nocera’s idea has great merit; just coming up with supplies of free hydrogen is worthwhile in itself. But a system of a water supply, a solar panel, hydrogen collection and storage, and a fuel cell plus a distribution of the electricity will come with substantial cost. Attractive as hydrogen fuel is compared to other storage methods, the focus needs to be on the production.
Hydrogen production is where Nocera’s work shines. The production is up to a point now where commercial interests might take some notice. By temporarily abandoning the poor and focusing on say the steps that upgrade the hydrogen into more useable fuels with an added carbon component such as methane or ethanol using the CO2 in the atmosphere would get production and commercial development going such that costs would go down getting the technology closer to the low income parts of the world.
Comments
4 Comments so far


[…] This post was mentioned on Twitter by Richard Walthers, Gas Diesel Prices. Gas Diesel Prices said: Sunlight and Water to Hydrogen and Electricity | New Energy and Fuel http://bit.ly/3795B1 […]
sadly you will never hear about this again
great post as usual!
Couldnt agree more with that, very attractive article